
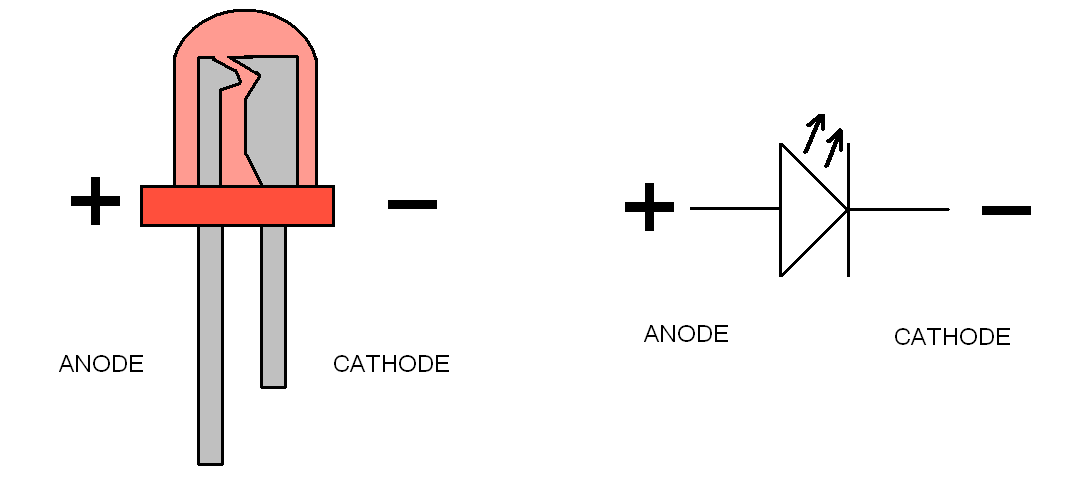
Using this equation, you can solve for n, mols of electrons.Current x time = mols of e - x Faraday's constant.Faraday's constant = coulombs of charge per mol of electron = total charge over total mols of electrons.Current = coulombs of charge per second.Faraday's law relating amount of elements deposited (or gas liberated) at an electrode to current.Without electrolytes, there won't be a circuit because electricity won't be able to travel.Electrolytes conduct electricity by the motion of ions.Anode shoots out electrons, Cathode takes in electrons.Cathode is always the place where reduction happens.Anode is always the place where oxidation happens.The following rules hold true for both electrolytic and galvanic/voltaic cells.Normally, the electrons would want to flow the other way (or not flow at all). In the diagram above, arrows are shown in red because the battery is forcing the flow of electrons.Thus, no input is required for galvanic/voltaic cells.
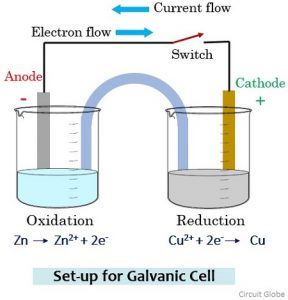
In contrast, galvanic/voltaic cells already have a positive cell potential.


 0 kommentar(er)
0 kommentar(er)
